
FITHI TEKESTE WELDEAB
Title: Using of Catalyst Carbon dioxide Hydrogenation To Manufacture Methanol
Area:
Country:
Program: Masters
Available for Download: Yes
View More Student Publications Click here
For more information on the AIU's Open Access Initiative, click here.
Table Of Contents
Abstract
1.Introduction
2.Literature Review
2.1 What is Methanol
2.2.History of Methanol Synthesis
2.3.Reaction Condition
2.3.1.Temperature
2.3.2.Pressure
2.3.3.Space Velocity
2.4.Catalyst
2.5.Pure Methanol as a motor vehicle fuel
2.5.1.Power
2.5.2.Driveability
2.5.3.Vapour Pressure
2.5.4.Driveability
2.5.5.Pure fuel – corrosion and compatibility
2.5.6.Pure fuel performance reliability and conversion
2.5.7.Carbon monoxide Emission
2.5.8.Hydrogen Emission
2.5.9.oxides of nitrogen Emissions
2.5.10.Aldehyde Emissions
2.5.11.Carbon dioxide to chemicals
2.6.Kinetics and Mechanism
3.Design flow Of Methanol Process
3.1.Methanol Process Description
3.1.1.Pre – reforming
3.1.2.Autothermic reaction
3.1.3.Separation Process
3.1.4.Compression
3.1.5.Methanol Synthesis
3.1.6.Purification
3.2.Methanol Production and Economy
3.2.1.Low Pressure Methanol Process
3.2.2.Liquid Phase Methanol Production [LPMEOH]
3.3.Methanol economy advantage compered to hydrogen economy
3.4.Methanol economy disadvantage
3.5.Methanol Health Effects
3.6.Methanol Toxicity
3.7.Hazards Identification
3.7.1.Effects of short-term (Acute) Exposure
3.7.2.Effects of long-term Exposure
4.Results and discussions
5.Conclusion
Reference
Figure.1. Methanol Flow Sheet.
Figure.2. steady state concentration with Varying temperature (P = 76.98 bar).
Figure.3. Products distribution (mole Fraction).
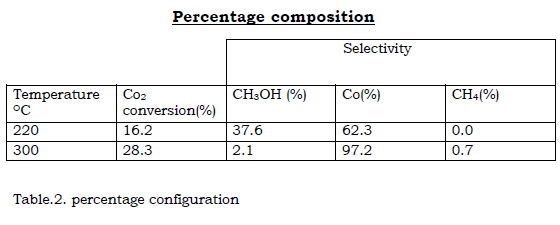
Table.1. Percentage configuration
ACKNOWLEDGEMENT
This project would not have been possible without the help and support of many people.
First and foremost, I would like to express my sincere grantitude to the almigth God for seeing me through thick and thin times during my study at this wonderful institution.
Seondly, I would like to thank my supervisor Montse Aullinace, for the continuous support he offered me during my M.SC. Studies, for his patience, motivation, enthusiasm, and immense Knowledge. His guidance helped me in all the time of my research and writing of this thesis. I couldn’t have imagined having a better advisor and mentor for my masters program.
Besides my advisor, I would like to thank my Co – supervisor Charles Davis,for this encouragement insightful comments, hard questions and for steering me towards the right direction all the time. My sincere thanks to all members of the department.
Last but not least, I would like to thanks my family; My Dady Tekeste, my mother Kidisti Ghede, and brothers and sister, fisha, shiden sinit. They support me spiritually and Financially to finish my studies.
Abstract
I studies the optimizing control for methanol plant. Natural gas was considered as the raw material for the special interest for both economic and political point view. Different technologies which are applied in practice for the production of the methanol mentioned were also studied in the literature review on the next page.
There are three main parts in the production of methanol namely:
1. Synthesis gas production, 2.methanol synthesis and 3.methanol purification.
But I choose the synthesis gas, because it is one of the best way of producing methanol in large capacity. It consists, pre – reforming process, Auto thermic reaction, separation process, compression, methanol synthesis and purification.
In this investigation I followed this process to get very pure and very efficient methanol. Methanol is not very difficult to manufacture, because I said that the raw material like Co, Co2 are easily we get it from industries and greenhouse effect.
I concluded from now on wards in this investigation if you can get easily the raw material that you have need Am sure you can do it with any problems of financial.
If the raw material that you have expensive, you get many challenges on your work, and financial crises etc.
CHAPTER ONE
1. Introduction
Carbon dioxide can be converted to methanol by combining it with hydrogen and using the right kind of catalyst. Of course, the hydrogen must be available on a large scale and it production must be achieved without any emission of Co2 to the atmosphere. This implies that the hydrogen must be produced by using renewable energies like solar, hydro or biomass fuel.
Methanol in addition to being a fuel on its own, can be looked at as a storage and transport medium for hydrogen. Methanol has also the advantage that it is liquid under normal conditions. It can be stored and transported as easily as gasoline and can be used in conventional combustion engines, without requiring any major adjustments. Methanol has twice the energy density of liquid hydrogen, and half the energy density of gasoline. Methanol synthesis can thus be looked up on as a way of converting hydrogen into an energy carrier that can be more conveniently stored and transported.
Therefore, advances in the chemistry of Co2 including its activation and utilization will be a necessary component of the development of a sustained economy in our society.
In process we can use homogenization methods of citric acid was selected in order to prepare the catalysts. The oxide catalysts containing CuO, ZnO, ZrO2 and one of the promoters La, Pd.
Methanol (MeOH) is one of the most important chemical feed stock used in the chemical industry, with a worldwide demand of approximately 50MTA [ Behrens et al 2012]. Methanol is used extensively in the plastics industry, as solvents in the pharmaceutical industry and on extraction of animal and plant products (as in the preparation of vitamins, cholesterol and hormones).
In organic synthesis, methanol is used as precursor of various chemical intermediates, mainly in the manufacture of formaldehyde. Additionally, it is considered as an excellent alternative energy resource.
For instance, more recently methanol has been considered as an alternative fuel when blended with gasoline and through the transition esterification of triglycerides to produce Biodiesel that reduce greenhouse gas emissions (olah, 2005).
It was also found that methanol can be converted into olefins in the MTO (Methanol- to- Olefins) process, into propylene in the MTP (Methanol – to – propylene) process (Koeple and liebner, 2007) and in turn, hydro carbon fuels can be produced through these olefins. Furthermore, the MTG and MTGD, processes, one produce hydrocarbons (Keil 1999). DMC (Dimethyl carbonate) synthesis and DME (Dimethyl ether) synthesis are other promising application of methanol.
Another aspect of environmental relevance is the mitigation of industrial emissions of carbon dioxide (Co2) through of Co2 capture by one process of hydrogenation. Hydrogenation of Co2 reduces the major man-made cause of global warming. However, it is worth noting that rendering effective Co2 reduction requires the use of low Co2 to methanol process stands as a promising alternative to Co2 reutilization due to the expanding of methanol.
In this direction, several heterogeneous catalysts for conversion of Co2 to methanol by catalytic partial hydrogenation have been investigated (High Field, 1995). Based on reported reaction mechanism of the Co/Co2/H2 conversion to methanol, three overall reaction [Bussehe and froment, 1996] occur over Cu/ZnO/Al2O3 catalysts.
Water gas shift: Co + 2H2 <-----> CH3OH
Methanol Steam reforming: Co2 + H2 <-----> Co + H2O
Co2 + 3H2 <-----> CH3OH + H2O
In reaction [1], methanol is produced by conversion of Co2 in an exothermic process. The resulting temperature increase enhances the rate of reaction. On the other hand, the resulting high temperature enhances the deteriorating effect of equilibrium conversion, resulting in the net methanol production decreasing.Hence the aim of present work is to the analysis of two CTM processes: production of methanol by hydrogenation of Co2 ( direct CTM, d – CTM) and production of methanol from synthesis gas (sg – CTM), sg – CTM is the conventional industrial process used in large scale worldwide. [K.Fujimoto, Y. Yu, Stud. Surf. Sci. Catal.(1993)].
CHAPTER TWO
2. Literature Review
2.1 What Is Methanol?
Methanol (also known as methyl alcohol and wood alcohol) is a colorless liquid that may explode when explode to open flame. It occurs naturally in wood and in volcanic gases. Methanol is also a product of decaying organic material. It is produced in large amounts (approximately 1.3 billion gallons 1992) by thirteen companies in the United States. US demand for methanol is likely to increase over the net several years. The largest users of methanol sold in the US are companies that make methyl t-butyl ether, a gasoline additive. Companies also use methanol to make chemicals such as formaldehyde, acetic acid, chloromethanes, and methyl methacrylate. Companies add methanol to paint strippers, aerosol spray paint, wall paints, carburetor cleaners and car wind shield washer products. Methanol is also a gasoline additive and in some cases, a gasoline substitute for use in automobiles and other small engines.
Exposure to methanol can occur in the workplace or in the environment following release to air, water, land or ground water. Exposure can occur when people use certain paint strippers, aerosol spray paint, wall paint, wind shield wiper fluid, and small engine fuel. Methanol enters the body when breathed in with contaminated air or when consumed with contaminated food or water. It can also be absorbed through skin contact. It does not remain in the body due to its breakdown and removal in expired air or urine.
[Wu – Hsun Cheng And Harold H.Kung Ed,(1994), Methanol Production And Use Chemical Industries, CRC Press].
2.2. History Of Methanol Synthesis
Methanol is believed to have been discovered by R. doyle in 1661 through the rectification of crude vinegar over milk of lime. He named the new compound adiaphorus spiritus lignorum. There was no written history or record of its use for any purpose, either domestic or industrial, before the 19th century.
The chemical or molecular identity of methanol was first established independently by J.B.A. Dumas and J.von liebig in 1834. The term “methyl” was introduced into chemistry in 1835 on the basis of their work.
Since then, efforts have been made by various investigators to synthesize methanol, and a successful attempt was the synthesis by dry distillation of wood, obtained by M.Berthelot in 1857. Referring to its synthesis origin and route, methanol has since been called “wood spirit”, by some people. Obviously this nick name is fading away due to its totally different synthesis route.
As early as 1913, A.Mittasch and co – workers at BASF successfully produced organic compounds containing oxygen, including methanol, from carbon monoxide and hydrogen in the presence of iron oxide catalysts during developmental work on the synthesis of ammonia. The decisive step in the large – scale industrial production of methanol was made by M.Pier and Coworkers in the early 1920s with the development of a sulfur – resistant zinc oxide chromium oxide catalyst. By the end of 1923 the process had been converted from the development to the production stage at the BASF leuna work. Processes based on the above work were performed at high pressure (250 – 350 bar ) and temperature ( 320 – 450OC). These processes defined methanol production for more than 40 years.
In the 1960s, however, ICI introduced a highly selective copper – zinc oxide catalyst, putting an end to the high pressure methanol synthesis technology. These catalysts operated at fairly mild reaction condition (50 – 100 bar and 200 – 300OC). Use of these more active catalysts was made possible because more efficient synthesis gas purification processes had become available, mainly removing the catalyst poisons ( metal carbonyls and sulfur).
[Wu – Hsun Cheng And Harold H.Kung Ed,(1994), Methanol Production And Use Chemical Industries, CRC Press].
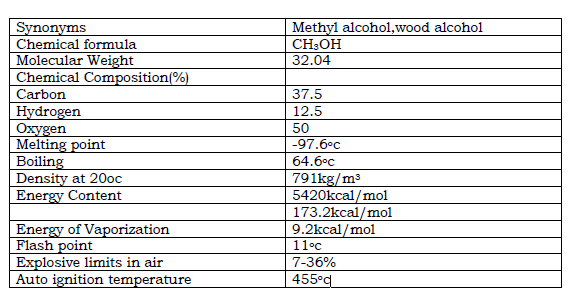 Table .1. Methanol Properties
Table .1. Methanol Properties
2.3 Reaction Condition
The main reaction conditions to be considered in methanol synthesis are temperature, pressure and space velocity.
[W.R.A.M. Robinson, (1989),“Structure And Activity Of Copper Containing Methanol Synthesis Catalysts”. University Of Amsterdam Thesis].
2.3.1. Temperature
Methanol synthesis is usually carried out at 493-573K. Since, hydrogenation reactions of Co and Co2 are exothermic; their rates increase with temperature but only up to a certain temperature.
At higher temperatures, the rates begin to decrease as the thermodynamic equilibrium constant decrease as temperature increases. Therefore, very high temperatures are not suitable. It was reported by bell et al.
That methanol yield increased with temperature but only up to 493K. Similarly it was found by Xin et al, that maximum Co2 conversion and yield were possible at around 523K. They also reported that methanol synthesis was more sensitive to reaction temperature than the water gas shift reaction.
Extreme temperature limit the efficiency of methanol production due to thermodynamic limitations. Therefore, low temperature route of methanol synthesis has been proposed by Tsubaki and coworkers. They conducted the experiments at 443K on copper based catalyst using ethanol as catalytic solvent. The showed that the reaction mechanism at low temperature followed: formate to methyl formate to methanol path way instead of formate to methoxy to methanol route. They proposed that low temperature methanol production enabled high conversions up to 50-80% and reduction of production cost without any thermodynamic equilibrium.
[W.R.A.M. Robinson, (1989),“Structure And Activity Of Copper Containing Methanol Synthesis Catalysts”. University Of Amsterdam Thesis].
2.3.2. Pressure
Methanol production was initially carried out at very high pressure when it was first started in 1920s. later lower pressure to 50-100 atm using a Cu/ZnO/Al2O3 catalyst. In 1988, Graaf et al, studied the kinetics of methanol synthesis form Co, Co2 and H2 over the same catalyst and developed a kinetic model operative at pressure of 15-50 atm.
They claimed their low pressure methanol synthesis kinetic model to be more precise in illustrating the experimental values compared to the previously proposed models. It was reported by deng et al, that methanol production could be carried at 20 atm using Cu/ZnO/Al2O3 catalyst.
[W.R.A.M. Robinson, (1989),“Structure And Activity Of Copper Containing Methanol Synthesis Catalysts”. University Of Amsterdam Thesis].
2.3.3.Space Velocity
Space velocity can have complicated effects on methanol yield. Xin et al, reported that both Co2 conversion and methanol yield decreased as space velocity was increased for a given value of Co2 concentration.
6
[W.R.A.M. Robinson, (1989),“Structure And Activity Of Copper Containing Methanol Synthesis Catalysts”. University Of Amsterdam Thesis].
2.4.Catalyst
Cu/Zno/Al2O3 is the catalyst mostly chosen for methanol synthesis due to its high selectivity, stability and activity. Copper acts as the main active component, Zno acts as a supporter and Al2O3 acts as a promoter. Another promoter used in the catalyst system is chromia. However, there are many controversies and questions regarding the individual catalyst components the role of ZnO and the identity of active sites. Most of the authors are of the view that metallic copper is the active component of the catalyst and the role of ZnO is to enhance dispersion of copper particles. Ovesen et al, concluded from their results that Cu was the active catalytic component in methanol synthesis.
Froment and Bussche also assumed Cu to be the active catalytic site and ZnO to provide structural promotion in the development of their detailed kinetic model for methanol formation. Another group of researchers led by Fujitani et al, However, have demonstrated conflicting results. They showed using surface science techniques that the role of ZnO was to form active sites in addition to dispersing Cu particles. Ostrovskii also reported that methanol synthesis occurs on the ZnO component of the catalyst.
Since, Co and Co2 hydrogenation is believed to occur on two different sites, it is proposed that doubts regarding the identity of active sites could be resolved. There are also efforts to develop novel catalysts that can effectively operate at lower temperature, lower pressure and exhibit water tolerance since water acts as an inhibitor for the catalyst.
[V.M. Cherednichenko, Korpova, Physico – Chemical Institute, Moscow, (1953)].
2.5 Pure Methanol As a Motor Vehicle Fuel
The use of either analytic or commercial grade methanol or methyl – fuel alone as a fuel for motor vehicles has also been suggested. As described in the preceding section, there are many problems associated with the use of blends of methanol and gasoline as a motor fuel, principally in the area of phase separation caused by the presence of water and the attendant alterations necessitated in the fuel distribution system. The separation problem is eliminated when pure methanol is used as a vehicle fuel; however, when this is done certain modifications to the vehicles itself become necessary. These modifications are considered to be more feasible particularly in the case where pure methanol is considered as the fuel fleets of vehicles.
2.5.1 POWER
For a stoichiometrically correct air the energy densities of the are very nearly equal (94.5 BTU/FT3 for methanol, 95.5 BTU/FT3 for gasoline) indicating that equal power can be extracted form comparable engines using these fuels.
Methanol will extract more brake – mean – effective pressure from an engine than will gasoline because of the increased volumetric efficiency afforded by the cooler methanol charge.
Equal power output has been achieved with methanol in an engine usually operated on gasoline when stoichiometric mixtures were used. Power output has been raised significantly above that for gasoline when richer mixtures were used; however, to gain the benefits in economy afforded by the lower lean misfire limit of methanol, this excess in power will have to be forfeited and possibly a sight loss in power sustained, for equivalent exhaust emissions, the methanol fuel engine has been shown to exhibit significantly higher power than the emission control equipped gasoline engine.
2.5.2 Driveability
Several of the driveability problems associated with the use of blends of methanol and gasoline are eliminated when pure methanol is used as a fuel; however, there remain problems associated with the vaporization properties of pure methanol as compared with those of gasoline.
2.5.3 Vapour Pressure
The vaporization characteristics of pure methanol have caused some problems when it is used as motor fuel. Its high heat of vaporization requires an enhanced supply of heat to the intake manifold in order to assure adequate mixture distribution to the cylinders. Its lower vapour pressure has made cold starting difficult and may necessitate the use of high volatility additives during this phase of the vehicle operation.
Its low boiling points requires careful attention to keeping the fuel lines and carburetor shielded from excess heat. All of these problems have been encountered and several solutions developed.
2.5.4 Driveability
The power output of a vehicle fuel with pure methanol can be equivalent to a comparable gasoline fuel vehicle and the solubility and separabilty problems of blends do not exist for pure methanol fuel. Most of the subjective evalutions of the driveability of vehicles fuel with pure methanol have focused on the problems associated with cold starting of such vehicles.
The higher heat of vaporization of methanol together with the requirement of over twice as much methanol as gasoline for the same amount of air to form astoichiometric mixture require that much more heat be supplied to the intake manifold to avoid cold starting and acceleration problems. Several solution to these problems have been proposed. It is possible to add high vapour pressure liquids or gases such as butane either generally or preferably only during cold start situations.
Ether gasoline or LPG could be injected at cold starts to accomplish the same effect. A side from the cold start problem, the performance of the methanol fuel vehicles has been shown to be equivalent to a gasoline fuel vehicles.
2.5.5 PURE FUEL – CORROSION AND COMPATIBILITY
Several corrosion and compatibility problems associated with the use of methanol in blends with gasoline as a motor fuel have been described. There has been much less experience reported concerning the use of pure methanol as a motor fuel. Many of the problems encountered with the use of blends may also appear during pure fuel use, but this has not been proven by experience.
9
Significant corrosion occurs after water causes separation of gasoline and methanol – water phase in blends. Much of this corrosion may be caused by the water the water in the lower phase. This separation does not take place when pure methanol is utilized as a motor fuel. Water is highly soluble in pure methanol and any traces found in the fuel system will be taken into the solution. The compatibility problems associated with the use of pure methanol as a motor fuel have been more extensively investigated. It may be expected that compatibility problems between pure methanol and Viton fuel system elements, metacrylate fuel filters and possibly certain types of fuel pump diaphragms and gaskets may exist.
The limited experience with the use of pure methanol as a motor fuel has uncovered some compatibility problems with certain fuel system components. Some test vehicles have suffered no corrosion or compatibility problems, and others have required alterations to avoid them. There is a need for further fleet testing in which the problems of corrosion and materials compatibility with pure methanol motor fuel are more completely investigated. It will only be through the experience gained during such fleet tests that all of these problems can be uncovered.
2.5.6 PURE FUEL - PERFORMANCE – RELIABILITY AND CONVERSIONS
The reliability of motor vehicles that have been converted to operation on pure methanol fuel has proven to be as high as that of comparable gasoline fuelled vehicles in several cases. The conversions necessary to enable a vehicle to operate with pure methanol as a fuel can be divided into two phases. Because the energy per cubic foot of stoichiometric mixtures of methanol and gasoline fuels is very similar, the modifications necessary to convert a conventional gasoline engine to pure methanol fuel are relatively simple. These conversions to enable the use of pure methanol fuel in conventional engines will be called first phase conversions. Such conversions involve changes to the carburetor, intake manifold, fuel system, and spark advance curve and do not require major engine modifications. This phase of engine modification may be easily carried out on a fleet of automobiles and has been done in several cases. In addition, major engine modifications such as increase in the compression ratio may be made an increase in fuel mileage. These modifications constitute a second phase of possible engine conversion. No full scale tests of such conversion have been reported.
Some modifications will be necessary to convert a conventional engine from gasoline to methanol fuel. Larger carburetor jets will be needed to provided the same range of operation. Its is also anticipated that some elastomeric seals in the fuel system may have to be changed depending up on their compatibility with methanol. Viton is the only material that has specifically caused problems in this area. Evaporative control cannisters and methacrylate fuel filters will have to be changed. Carburetor or fuel tank substitutions may be necessary on certain vehicles because of corrosion problems. The possible corrosion and compatibility problems are not well defined and require an enlarged fleet test program to uncover them.
Some means of assuring fuel vaporization and even distribution to the cylinder will have to be provided. This can be accomplished by placing heat exchangers in the intake manifold or by the adoption of a fuel injection system. Provision will be necessary to assist in cold starting engines injection of butane, propane, acetone, ethyl ether, gasoline, or LPG during the starting procedure. The adoption of fuel injection would benefit in this area as well. Depending up on the particular vehicle and its fuel system layout there may be problems with vapour lock during hot operation. The installation of an electric fuel pump should eliminate these.
2.5.7 CARBON MONOXIDE EMISSION
Since the methanol molecule has no carbon to carbon bonds and already contains one oxygen atom, the reaction kinetics for complete oxidation of this fuel are theoretically less complex than those for gasoline and the intermediate reaction products that form exhaust emissions are more readily eliminated from the exhaust system. In addition it has been shown earlier that methanol fuelled vehicles will operate satisfactorily at much leaner mixture ratios. Therefore, it may be expected that vehicles fuelled by pure methanol, especially those equipped with catalytic muffler will emit less carbon monoxide than comparable gasoline fuelled vehicles. This reduction has been measured except in these cases in which operation was fuel- rich. The extended fuel – rich warm-up period necessary with methanol fuelled vehicles appreciably raises their Co emissions. The installation of catalytic converter has been shown to reduce Co emissions below the 1977 federal standard.
2.5.8 HYDROCARBON EMISSION
Several tests have been performed on methanol fuelled vehicles from which the emissions of hydrocarbons emitted in the exhaust of methanol fuelled vehicles are different than those emitted by gasoline fuelled vehicles and adequate provisions must be made to insure that recording equipment accurately measures their presence. The major component of unburned fuel in the exhaust has been found to be methanol which is technically not a hydrocarbon at all; however, the emissions of unburned fuel are generally reported under the heading of hydrocarbon emissions.
The lower operation afforeded by the use of methanol fuel should lead to lower hydrocarbon (HC) emissions. The emissions of hydrocarbons and unburned fuel from engines fuelled by methanol have been shown to be lower than those fuelled by gasoline during hot start emissions tests. Because of the longer warm-up period under fuel rich operation which is necessary with methanol fuel, the HC emission from methanol fuel were higher than these from gasoline during cold start tests.
The use of a catalytic muffler was found to be necessary in order to meet the 1977 federal HC standard. These are no armaties emitted among the hydrocarbons in the exhaust of a methanol fuelled vehicle and there is also expected that the total reactively caused by the unburned fuel for the formation of photochemical air pollution is much lower for a methanol fuelled vehicle than for gasoline.
2.5.9 OXIDES OF NITROGEN EMISSIONS
The emissions of oxides of nitrogen (NOx) in the exhausts of methanol fuelled vehicles have been demonstrated to be very low; lower than the NOx emissions of comparable gasoline fuelled vehicles. Emission levels below the 1977 federal NOx standard have been demonstrated with methanol fuelled vehicles without the use of emissions control equipment. Results have shown no increase in NOx emissions during cold start tests. Calculations have shown that the peak otto cycle temperature of methanol fuel is lower than that of iso-octane. The lower combustion temperature of methanol contributes to the depression of NOx emissions from methanol fuelled vehicles. The higher flame velocity exhibited by methanol as compared to that of gasoline allows the use of later spark timing which also results in lower NOx emissions.
Since emissions of NOx peak at stoichiometric conditions of operation, the lower lean misfire limit exhibited by methanol permits lower NOx emissions by allowing operation at much leaner mixture ratios than gasoline.
2.5.10 Aldehyde Emissions
Aldehydes form a class of potential air pollutants that are not presently covered by federal standards. The presence of certain aldehydes, principally formaldehyde and acetaldehyde has been measured in exhaust of methanol fuelled engines. Aldehyde emissions from automobiles have not been measured as extensively as those of other air pollutants. There exists no federal standard for aldehyde emissions. The level of aldehyde emissions from methanol fuelled vehicles seems to be a sensitive function of the air to fuel mixture ratios. Some tests have shown that the aldehyde emission level from methanol fuelled vehicles. Others have shown increased aldehyde emissions when methanol is substituted for gasoline as a fuel. There is a need for further testing of both methanol fuelled vehicles and gasoline fuelled vehicles, however, before any definite conclusions can be drawn. The use of a catalytic muffler has been shown to considerably reduce the aldehyde emissions of methanol fuelled automobiles.
2.5.11 Carbon dioxide to chemicals
Carbon dioxide is a major greenhouse gas, however it can also be considered as an important carbon source to produce valuable chemicals and fuels via carbon dioxide hydrogenation, carbon dioxide cyclo addition to epoxides, Co2 carbonylation of amines or alcohols, and the like (Daietal, 2009; razali et al, 2012). Several innovative technologies as selectively transform Co2 into highly valuable materials by alternating co polymerization ion of Co2 with other organic compounds, have been developed for industrial applications and are accessible to markets. An excellent review by song (2006) had addressed the barriers, strategic objective and approaches for Co2 capture and utilization. The barriers for Co2 utilization are as follows: (1) cost of ccs techniques; (2) energy requirement in Co2 conversion; (3) market scale; (4) Socio- economical driving force. The strategies of Co2 utilization should focus on the use of Co2 for the environmentally benign process, the production of industrially useful chemicals from Co2 and the Co2 recycling involving renewable energy to conserve carbon sources.
In view of thermodynamics of Co2 conserve, for example, gibbs free energy of Co2 and related substances, many reactions exhibit positive change in enthalpy (ΔH>0) and thus are exothermic. In other words, low energy input, active catalysts and effective reaction conditions are demanded for conversion of Co2 to chemicals. One feasible and thermodynamically practical route is to use Co2 as a Co- reactant to react with anther substance that possesses higher Gibbs free energy, for example, hydrogen and methanol. Omae (2012) extensively reviewed related products (carbonates) from Co2 with various types of reactive substances including oxygen – containing compounds, nitrogen – containing compounds, carbon – carbon unsaturated compounds, and others substances such as hydrogen.
Aresta and dibenedetto (2007) comprehensively reviewed the interactions and the bonding modes of Co2 with metal centres of catalysts. Various modes of bonding were generated according to different metal centers of catalyst, thus could drive different reaction based on Co2 and further resulted in different structural features of compounds. They also reviewed the incorporation of Co2 as a building block into an organic substrate via the following bonding: (1) C-N bond to afford acids, esters, e.t.c; (2) C-N bond to offord carbamates and isocyanates; (3) C-O bond to afford cyclic, linear, and poly carbonates.
The use of catalyst is generally required to transform Co2 to organics under mild conditions. Numerous and heterogeneous catalysts have been proposed. Both homogeneous and heterogeneous catalysts possess advantages and disadvantages. Homogeneous catalysts typically show higher catalytic activity and selectivity than heterogeneous catalysts under mild conditions. Heterogeneous catalysts, however, have superior stability and durability and provide simplicity in separation, handling and reactor design, thus are preferred in Co2 conversion (Dai etal, 2009; Razali etal, 2012).
2.8 Kinetics And Mechanism
The mechanism of methanol synthesis over Cu/Zno/Al2O3 the catalysts used in the commercial low pressure process has been under discussion for several years. Questions that have pervaded the literature are the reactive carbon containing component (Co or Co2) and the overall mechanism.
Mixtures of both H2/Co and H2/Co2 will form methanol over a Cu/Zno/Al2O3 catalyst and it was early believed that methanol synthesised from a mixture of H2/Co/Co2 was the product of Co hydrogenation (I. wender, Reactions of synthesis gas, Fuel Processing Technology, 1996). However, it was discovered that the methanol synthesis was promoted by addition of small amounts of Co2 to the H2/Co mixture while large Co2/Co ratios inhibited the synthesis, thus providing a maximum in the reaction rate. This maximum was first concluded to exist due to an oxidized state and a reduced state of the catalyst, which could be controlled by the Co/Co2 ratio in the feed ( Equation 1).
The intermediate reduced state is inactive and the oxidized state is active. The increase in reaction rate at high ratio of Co/Co2 explained by excessive reduction of the catalyst, inactivation of the sites, while at low ratios it were thought to be due to strong adsorption by Co2. A mechanism was proposed building on an intuitive Co hydrogenation with a Co2/Co redox – mechanism.
Aox + Co(g) <----> Ared + Co2 (g) ---1
Based on these observations a rate expression was build up by two terms; the first is the main term and relates the complete adsorption of Co2 and Co or H2 to the synthesis of methanol from Co under high pressure. The second term corresponds to the reaction directly from Co2.
This equation states that the reaction rate is zero for gases which contains no Co2 (equation -2).
ƔCH3OH= Const K3 redox (Pco2/Pco)3 (PcoPH22 – PCH3OH/K2)+K(Pco2-(1/k)(PCH3OH Pco/PH23))
1+ Kredox (Pco2/Pco)3 (f + Kco2 + Pco2)n
Equation -1
Though modelling of the methanol synthesis villa etal. Found that the water – gas – shift reaction also should accounted for in the modelling of the system, which was not accounted for in equation 1.
The set of equations ( 2,3 ) proposed assumes that Co is the carbon containing source of methanol and that the water gas shift reaction and hydrogenation of carbon monoxide occurs on different catalytic sites.
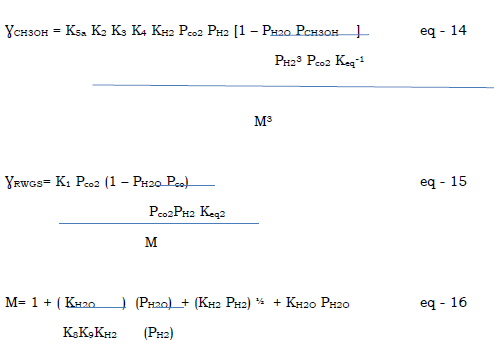
ƔCH3OH = FcoFH22 – FCH3OH/K2 eq – 2
(A + Bfco + CfH2 + Gfco2)3
ƔWGS = Fco2FH2 – FcoFH2OK3 eq - 3
M2
Evidence that the actual reactant in methanol synthesis is Co2 was obtained using isotope labelled Co2 in the feed. At high space velocity the methanol product had similar content as the Co2 fed to the reactor. Due to interference from intermediate reactions in the water – gas – shift reaction, the results for low space velocity did not have the same carbon containing intermediate on the catalyst surface.
Chinchen and spencer proposed a mechanism in which methanol and adsorbed oxygen was generated on the copper surface through hydrogenation of Co2 and involving the formation of formats and their hydrogenolysis to methoxy groups. Since Co is a better reducing agent than H2 it resulted in a linear relationship between Co2/Co ratio and oxygen coverage and the adsorbed oxygen would promote chemisorption of Co2. Coverage of oxygen or chemisorbed species, which could explain the inhibition from large Co2/Co ratios.
H2 (g) --------- 2H eq - 4
Co2 (g) ------- Co2 eq - 5
Co2 + H ------ HCo2 eq - 6
HCO2 + 2H ------- CH3O eq - 7
CH3O + H -------- CH3OH(g) eq - 8
Co(g) + O -------- Co2(g) eq - 9
2H(g) + O ------- H2O(g) eq - 10
Despite the results of chinchen and spencer, Graaf et al. derived kinetic expressions based on the hydrogenation of Co and Co2 together with the reverse water – gas- shift reaction. A dual – site Langmuir – Hinshelwood mechanism was proposed, which consisted of the adsorption of Co and Co2 on one site while H2 and H2O adsorbs on another site.
 This model predits that there are two different concentration of one of the same species, like formyl and methoxy, since same intermediates occur in two different overall reactions.
(Vanden Bussche and Froment , ASteady State Kinetic Model For Methanol Synthesis And The Water Gas Shift Reaction On A Commercial Cu/Zno/Al2O3 Catalysts, Journal of Catalysis, 1996). Couple the rate of both overall reactions through adsorbed oxygen intermediate, which also account for the water gas shift reaction, using a langmuir – Hinselwood Hougen – Watson mechanism. The main source of carbon in methanol is assumed to be Co2 and Co is the source of Co2 through the water gas shift reaction.
This model predits that there are two different concentration of one of the same species, like formyl and methoxy, since same intermediates occur in two different overall reactions.
(Vanden Bussche and Froment , ASteady State Kinetic Model For Methanol Synthesis And The Water Gas Shift Reaction On A Commercial Cu/Zno/Al2O3 Catalysts, Journal of Catalysis, 1996). Couple the rate of both overall reactions through adsorbed oxygen intermediate, which also account for the water gas shift reaction, using a langmuir – Hinselwood Hougen – Watson mechanism. The main source of carbon in methanol is assumed to be Co2 and Co is the source of Co2 through the water gas shift reaction.
 [ Erik Sjoberg, Sept 2009, Membrane Processes For Effective Methanol Synthesis In The Forest Based Bio Refining, Sweden].
In haled methanol is absorbed for 60 – 85% through the, especially upper, respiratory tract, with not much difference between humans and animals. After dermal exposure, methanol rapidly diffuses through the skin. In human volunteer study, a dermal penetration rate of 8.1mg/cm2/h has been established.
Results from toxicokinetic experiments and modeling indicate that blood methanol levels in rodents and primates increase linearly at 8 – hour exposures up to ca. 1600mg/m3 (1200ppm), At higher levels, blood methanol levels increase linearly in humans, but non – linearly in laboratory animals (most sharply in mice, less sharply in monkeys). At exposures to ca. 266mg/m3 (200ppm), levels in rats and monkeys do not exceed endogenous levels.
Methanol distributes through the body uniformly to body water content. In the liver, methanol is metabolised through formaldehyde (invodents by catalase, in primates by alcohol dehydrogenase) and formate to carbon dioxide.
[ Erik Sjoberg, Sept 2009, Membrane Processes For Effective Methanol Synthesis In The Forest Based Bio Refining, Sweden].
In haled methanol is absorbed for 60 – 85% through the, especially upper, respiratory tract, with not much difference between humans and animals. After dermal exposure, methanol rapidly diffuses through the skin. In human volunteer study, a dermal penetration rate of 8.1mg/cm2/h has been established.
Results from toxicokinetic experiments and modeling indicate that blood methanol levels in rodents and primates increase linearly at 8 – hour exposures up to ca. 1600mg/m3 (1200ppm), At higher levels, blood methanol levels increase linearly in humans, but non – linearly in laboratory animals (most sharply in mice, less sharply in monkeys). At exposures to ca. 266mg/m3 (200ppm), levels in rats and monkeys do not exceed endogenous levels.
Methanol distributes through the body uniformly to body water content. In the liver, methanol is metabolised through formaldehyde (invodents by catalase, in primates by alcohol dehydrogenase) and formate to carbon dioxide.
In rodents, the rate – limiting step in the metabolism of methanol is the oxidation of methanol to format, while the oxidation of formate to carbon dioxide is rate limiting in primates.
As a consequence, exposure to high concentration or doses of methanol may cause accumulation of methanol in rodents and format in primates. In humans, accumulation of formate may occur at methanol doses > 210 mg/kg bw.
By far the most of the methanol taken up and distributed is excreted as carbon dioxide and only minor amounts uncharged by the lungs and the kidney. The methanol metabolites formaldehyde and format are thought to bind to various endogenous molecules or enter a number of endogenous synthetic path ways. In humans, elimination half – lives of methanol in blood and in urine and breath ca.
The most suitable biological parameter for biological monitoring of persons exposed to methanol is the methanol concentration in urine.
CHAPTER THREE
3. Design Flow Of Methanol Process
3.1. Methanol Process Description
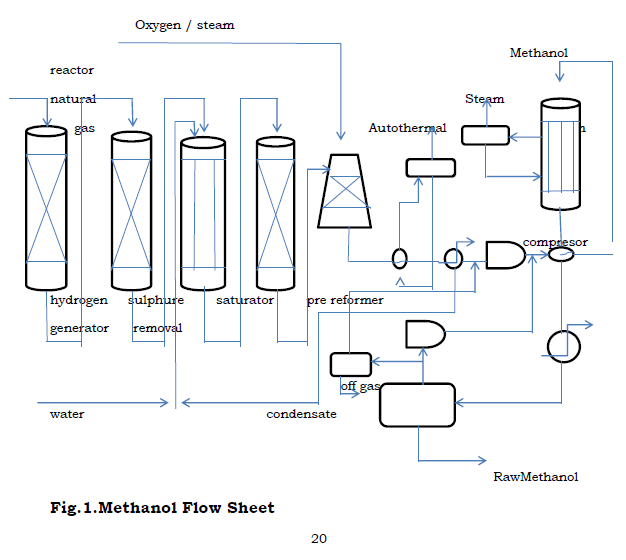 3.1.1.Pre – reforming
3.1.1.Pre – reforming
Pre – reforming is the term that has been applied to the low temperature steam – reforming of hydrocarbons in a simple adiabatic reactor. The pre – reformer utilizes the heat content of the feed stream to drive the steam reforming reaction at low temperatures. This reactor also uses nickel catalyst to promote the rate of the reaction. This pre – reformer is able to convert the higher hydrocarbons into methane and carbon dioxide. It operates at a temperature of about 497oc. The pre – reforming reactions result in an equilibrium gas mixture containing hydrogen, carbon monoxide, carbon dioxide, methane and steam.
[V.M. Cherednichenko, Korpova, Physico – Chemical Institute, Moscow, (1953)].
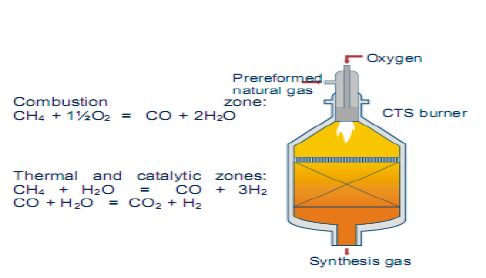
3.1.2. Auto thermic Reaction [ATR]
ATR operates at low steam to carbon ratio and the development of new burner designs ensures safe operation and high on – stream factors. The alternative measurement to achieve lower H2/Co ratios is the addition of oxygen. Autothermic reforming is the reforming of light hydrocarbons mixture of stream and oxygen in the presence of a catalysts. The oxidation reaction is used to adjust a synthetic ratio. In this process, the autothermal reforming processes was used to produce synthesis gas. The reaction occurring in the ATR reactor:
CH4 + 1.5 O2 <---> CO + 2 H2O
CH4 + H2O <---> CO + 3 H2
CO + H2O <---> H2 + CO2
[V.M. Cherednichenko, Korpova, Physico – Chemical Institute, Moscow, (1953)].
 3.1.3. Separation process
3.1.3. Separation process
Since all the reactions occurring in the ATR are exothermic reactions, the temperature of the product is very high. The product need to be cooled to a lower temperature before the separation can take place. After cooling the products, it is then separated into, the synthesis gas part leaving at the top of the separator whiles the water in the mixture leaves at the bottom.
[V.M. Cherednichenko, Korpova, Physico – Chemical Institute, Moscow, (1953)].
3.1.4. Compression
The pressure of the synthesis gas emanating from the separator is increase from 30 bar to 80 bar and this is done by using a compressor.The compressed mixture is then mixed with a recycle stream from the flash drum as shown in the flow sheet. The temperature of the resulting mixture is then raised to 270oc before it enters the methanol reator.
[V.M. Cherednichenko, Korpova, Physico – Chemical Institute, Moscow, (1953)].
3.1.5. Methanol Synthesis
The make – up synthesis gas and the recycle at (270oc, 80 bar) contains mostly hydrogen, carbon monoxide and carbon dioxide. The main reactions for the methanol formation are hydrogenation of CO, hydrogenation of CO2 and then coupled with the reverse water gas shift reaction. Methanol is thermodynamically less stable and therefore the catalyst used should be very selective. The three reactions are as follows:
CO + 2H2 <---> CH3OH
CO2 + 3H2 <---> CH3OH + H2O
CO2 + H2 <---> H2O + CO
Two independent reactions (hydrogenation of carbon monoxide and the reverse water gas shift) were considered out of the three dependent reactions. The rate of reaction constants combined with the equilibrium rate constant provides enough information about kinetics of methanol synthesis. The reaction rate constants, adsorption equilibrium constants and reaction equilibrium constant.
The reactor effluent is cooled to 40oc before it is sent to the vapour – liquid separator. Gas / liquid separation is carried out in a vessel under pressure. The gas is recycled after purging a small part to keep the loop within limits.
[V.M. Cherednichenko, Korpova, Physico – Chemical Institute, Moscow, (1953)].
3.1.6. Purification
The water – methanol mixture is distilled in order to meet the final specifications. It is essential for methanol to be stabilized (either by distillation or by deep flashing) on order to remove volatile components such as CO2 and permit shipment and transport in atmospheric vessels.
There are three grades of methanol namely: chemical grade AAA ( 99.85 wt% MeOH, 0.1 wt% water and concentrations of higher alcohol at parts – per – million levels), fuel grade ( 97 wt% MeOH, 1 wt% water, 1.5 wt% alcohols and 0.5 wt% of process oil) and MTBE grade ( 97 wt% MeOH, 1wt% water, 2 wt% alcohols, 150 ppm methyl acetate, 0.3 wt% inert liquid medium).
V.M. Cherednichenko, Korpova, Physico – Chemical Institute, Moscow, (1953)].
3.2. Methanol Production and Economy
Methanol is produced by the hydrogenation of carbon dioxides over a suitable ( copper oxide, zinc oxide, or, chromium oxide based) catalyst.
Co + 2H2 <---> CH3OH
Co + 3H2 <---> CH3OH + H2O
The first reaction is the primary methanol synthesis reaction, a small amount of Co2 in the feed (2-10%) acts as a promoter of this primary reaction and helps maintain catalyst activity. The stoichiometry of both reaction is satisfied when R in the following relation is 2.03 minimally (katofsky 1999). H2 build up in the recycle loop, this leads to an actual R value of the combined synthesis feed (make up plus recycle feed) of 3 to 4 typically.
R= H2 – Co2
Co + Co2
These reactions are exothermic and give a net decrease in molar volume. Therefore the equilibrium is favoured by high pressure and low temperature. During production, heat is released and has to be removed to keep optimum catalyst life and reaction rate.
The catalyst deactivates primarily because of loss of active copper due to physical blockage of the active sites by large – product molecules; poisoning by halogens or sulphur in the synthesis gas, which irreversibly form inactive copper salts and sintering of the copper crystallites into larger crystals, which then have a lower surface to volume ratio.
Conventionally, methanol production first began in 1923 at BASF’s leuna, Germany Plant. The process required a high pressure (250 – 350 atm) and catalyst selectivity was poor.
In the 1960s and 1970s then more active Cu/Zn/Al catalyst was developed allowing more energy – efficient and cost-effective plants and larger scales. Processes under development at present focus on shifting the equilibrium to the product side to achieve higher conversion per pass.
Examples are the gas/soild/solid trickle flow reactor, with a fine adsorbent powder flowing down a catalyst bed and picking up the produced methanol and liquid. Fundamentally different could be the direct conversion of methane to methanol, but despite a century of research this method has not yet proved its advantages.
[S.PS. Andrew, Paper 12, Post Congress Symposium, 7th International Congress On Catalysis, Osaka, (1980)].
The methanol needed in the methanol economy can be synthesized not only from a wide array of carbon sources including still available fossil fuels and biomass but also Co2 emitted from fossil fuel burning power plants and other industries and eventually even the Co2 contained in the air.
Today most methanol is produced from methane through syngas. Through conventional natural gas resources are currently the preferred feed stock for the production of methanol, un conventional gas resources such as coal bed methane, tight sand gas and eventually the very large methane hydrate resources present under the continental shelves of the seas and Siberian and Canadian tundra could also be used. Besides methane all other conventional or un conventional fossil fuels could be utilized to produce methanol.
Besides the conventional route to methanol from methane. Passing through syngas generation by steam reforming combined with partial oxidation, new and more efficient ways to produce methanol from methane are being developed. These include:
-Methane oxidation with homogenous catalysts in sulphuric acid media
-Methane bromination followed by hydrolysis of the obtained bromomethane.
-Direct oxidation of methane with oxygen.
-Microbial or photochemical conversion of methane.
The use of methane or another fossil fuel for the production of methanol using all the above – mentioned synthetic routes has a potential drawback: the emission of the greenhouse gas carbon dioxide. To mitigate this, methanol can be made through ways minimizing the emission of Co2. One solution is to produce it from syngas obtained by biomass gasification.
For this purpose any biomass can be used including wood, wood waste, aquatic plants and municipal waste. There is no need to use food crop as in the case of ethanol from corn, sugar cane and wheat.
Biomass --> syngas (Co,Co2,H2) --> CH3OH
More importantly, methanol can be also be produced from Co2 by catalytic hydrogenation of Co2 with H2 where the hydrogen has been obtained from water electrolysis. This is the process used by carbon recycling international of ice land. Methanol may also be produced through Co2 electrochemical reduction, if electrical power is available. The energy needed for these reactions in order to be carbon neutral would come from renewable energy sources such as wind, hydroelectricity and solar as well as nuclear power. In effect, all of them allow free energy to be stored in easily transportable methanol, which is made immediately from hydrogen and carbon dioxide, rather than attempting to store energy in free hydrogen.
Co2 + 3H2 CH3OH + H2O
Co2 + 2H2O + electrons --> Co + 2H2 (+3/2 O2) --> CH3OH
The necessary Co2 would be captured from fossil fuel burning power plants and other industrial fuel gases, including cement factories. With diminishing fossil fuel resources and therefore Co2 emissions, the Co2 content in the air could also be used. Considering the low concentration of Co2 will have to be developed. This would allow the chemical recycling of Co2, thus mimicking nature’s photosynthesis.
3.2.1. Low Pressure Methanol Process
Conventional methanol reactors (cybulski 1994, kirk-oithmer 1995) use fixed beds of catalyst pellets and operate in the gas phase. Two reactor types predominate in plants built after 1970. The ICI low-pressure process is an adiabatic reactor with cold unreacted gas injected between the catalyst beds. The subsequent heating and cooling leads to an inherent inefficiency, but the reactor is very reliable and therefore still predominant. The lurgi system with the catalyst loaded into tubes and a cooling medium circulating on the outside of the tubes, allows near-isothermal operation.
Conversion to methanol is limited by equilibrium consideration and the high temperature sensitivity of the catalyst. Temperature moderation is achived by recycling large amounts of hydrogen rich gas, utilising enhance the heat transfer. Typically a gas phase reactor is limited to about 16% Co gas in the inlet to the reactor, in order to limit the conversion per pass to avoid excess heating.
The methanol synthesis temperature is typically between 230 and 2700C the reactor operates adiabatic. The pressure is between 50 and 150 bar. Higher pressure give economic benefit, since the equilibrium than favours methanol. Only a portion of the Co in the feed gas is converted to methanolin one pass through the reactor, due to the low temperature at which the catalyst operates. The unreacted gas is recycled at ratio typically between 2.3 and 6. The copper catalyst is poisoned by both sulphur and chlorine but the presence of free zinc oxides does help prevent poisoning.
[S.PS. Andrew, Paper 12, Post Congress Symposium, 7th International Congress On Catalysis, Osaka, (1980)].
3.2.2. Liquid phase Methanol Production [LPMEOH]
In liquid phase processes (cyulski 1994, USDOE 1999) heat transfer between the solid catalyst and the liquid phase is highly efficient there by allowing high conversions to be obtained without loss of catalyst activity. Higher conversion per pass eliminates the need for a recycle loop, which would imply less auxiliary equipment, less energy requirements, smaller volumetric flow through the reactor (Katofsky 1993).
Different reactor types are possible for liquid phase methanol production, such as a fluidised beds and monolithic reactors. The slurry bubble column reactor of the LPMEOH (registered trademark of Air products and chemicals,INC) process is used in this study. The LPMEOH process was invented in the late 1970s and further developed and demonstrated in the 1980s by Air products.
Since the 1990s a commercial scale demonstration is taking place at Eastmans king sport, tenessee, chemicals – from – coal complex. The demonstration is a co-operation of Air products, Eastman chemicals and the US department of energy.
The main difference of the LPMEOH process compared to fixed bed process are:
•Better heat transfer and therefore excellent temperature control with smaller heat exchangers.
•The finely divided catalyst allows for efficient use of available surface and therefore faster mass transfer.
•Higher conversion per pass.
•More even process temperature, which can be positively affect the stability of catalyst, being a strong function of temperature.
•Easy and rapid accommodation to change in feed rate and composition without operational problems or catalyst damage.
•The ability to withdraw spend catalyst and add fresh without interrupting the process.
Commercial Cu/Zn/Al catalyst developed for the two-phase process are used for the three –phase process. The powdered catalyst particles typically measure 1 to 10μm and are density suspended in a thermo stable oil, chemically resistant to components of the reaction mixture at process conditions, usually parafine catalyst deactivation due to exposure to trace contaminants is still a point of concern (cybulski 1994).
[S.PS. Andrew, Paper 12, Post Congress Symposium, 7th International Congress On Catalysis, Osaka, (1980)].
3.3. Methanol Economy Advantages Compared to hydrogen Economy:
Efficient energy storage by volume, as compared with compressed hydrogen, and when hydrogen pressure – confinement vessel is taken into account, an advantage in energy storage by weight can also be realized. The volumetric energy density of methanol is considerably higher than liquid hydrogen, input because of the low density of liquid hydrogen of 71 grams/liter. Hence there is actually more hydrogen in a litre of methanol (99 grams/liter) than in a liter of liquid hydrogen and methanol needs no cryogenic container maintained at temperature of 2530C.
A liquid hydrogen infrastructure would be prohibitively expensive methanol can use existing gasoline infrastructure with only limited modifications.
Can be blended with gasoline (for example a mixture containing 85% methanol and 15% gasoline).
User friendly, hydrogen is volatile and requires high pressure or cryogenic system confinement.
Less losses: making storage systems hydrogen leakage proof is a lot more difficult than preventing leakage from methanol. With liquid hydrogen heat will evaporate liquid, giving expected losses up to 0.3% per day in storage tanks due to release of over pressure.
[Sigurd Skogestad,(2000), Plant Wide Control: The Search For The Self Optimizing Control Structure. “Journal Of Process Control”].
3.4. Methanol Economy Disadvantages
• High energy costs associated with generating hydrogen (when needed to synthesize methanol).
• Depending on the feed stock the generation in itself may be not clean.
• Presently generated from syngas still dependent on fossil fuels (although in theory any energy source can be used).
• Energy density (by weight or volume) one half of that of gasoline and 24% less than ethanol.
• Corrosive to some metals including aluminum, zinc and manganese. Similar to ethanol, compatible material for fuel tanks, gasket and engine intake have to be used.
[Sigurd Skogestad,(2000), Plant Wide Control: The Search For The Self Optimizing Control Structure. “Journal Of Process Control”].
3.5. Methanol Health Effects
Methanol is a color less liquid with a mild alcohol Oder. It is widely used as a chemical feed stock to produce a variety of consumer products. While consumer exposure to methanol should be avoided and will continue to be minimized with the use of well-engineered fuel containers meeting stringent requirements, it is useful to review the health effects of exposure to methanol.
Methanol is toxic to humans and is readily absorbed by ingestion and in halation and more slowly by skin exposure. However, methanol is already present with in the human body in small quantities from eating fruits and vegetables. According to the FDA, as much as 500 milligrams per day of methanol is safe in adult’s diet. In the body, methanol is metabolized in the liver, converted first to formaldehyde, and then to formate. As a building block for many biological molecules, formate is essential for survival.
High levels of formate build up after excessive methanol intake, however can cause severe toxicity and even death. Refueling a fuel cell car with methanol will only give low-dose exposure (23 – 30 PPM for a few minutes), with a small intake of 3 milligrams of methanol. This is less than drinking a single can of diet soda containing 200milligrams of aspartame an artificial sugar containing methanol, which would produce 20 millgrams of methanol in the body.
The initial symptoms of methanol poisoning (drinking one to four ounces) may be delayed for as long as 12 to 18 hours as the body metabolizes methanol to formate and can consist of weakness dizziness, head ache, nausea vomiting and blurred vision. In several cases of accidental or reckless ingestion, methanol poisoning may lead to permanent blindness or death, although complete recovery is the rule in patients admitted early to a hospital. There are several treatments available to combat methanol poisoning, including early treatment with sodium bicarbonate to help prevent visual impairment. In a hospital setting, hemodialysis is effective in removing both methanol and formate from the blood and co-exposure to ethanol has been shown to reduce formate levels.
In case of skin exposure to methanol, washing immediately with soap and plenty of water can prevent further skin absorption.Methanol exposure should be avoided and can be managed safely through the proper design of fuel containers and fueling systems. A spill free nozzle has been developed by identic of Sweden that features a dry connection to the fuel cell that makes it virtually impossible for the consumer to contact methanol from the pump or the vehicle.
[Sigurd Skogestad,(2000), Plant Wide Control: The Search For The Self Optimizing Control Structure. “Journal Of Process Control”].
3.6. Methanol Toxicity
Methanol occurs naturally in the human body and in some fruits, but is poisonous in high concentration. Ingestion of 10ml can cause blindness and 60-100ml can be fatal, if the condition is untreated. Like many volatile chemicals, methanol does not have to be swallowed to be dangerous since the liquid can be absorbed through the skin, and the vapour through lunges.
Methanol fuel is much safer when blended with ethanol, even at relatively low ethanol percentages. US maximum allowed exposure in air (40h/week) is 1900mg/m3 for ethanol, 900mg/m3 for gasoline, and 1260mg/m3 for methanol. However, it is much less volatile than gasoline and therefore has lower evaporative emissions, producing a lower exposure risk for an equivalent spill. While methanol offers somewhat different toxicity exposure pathways, the effective toxicity is no worse than those of benzene or gasoline, and methanol poisoning is far easier to treat successfully. One substantial concern is that methanol poisoning generally must be treated while it is still a symptomatic for full recovery.
In halation risk is mitigated by a characteristic pungent odor. At concentration greater than 2000ppm (0.2%) it is generally quite noticeable, however lower concentration may remain undetected while still being potentially toxic over longer exposures and may still present a fire/explosion hazard. Again, this is similar to gasoline and ethanol; standard safety protocols exist for methanol and are very similar to those for gasoline and ethanol.
Use of methanol fuel reduces the exhaust emissions of certain hydrocarbon related toxins such as benzene and 1,3 butadience and dramatically reduce long term ground water pollution caused by fuel spills. Un like benzene – family fuels, methanol will rapidly and non-toxically biodegrade with no long term harm to the environment as long as it is sufficiently diluted.
[Sigurd Skogestad,(2000), Plant Wide Control: The Search For The Self Optimizing Control Structure. “Journal Of Process Control”].
3.7 Hazards Identification
3.7.1 Effects of short – term (Acute) Exposure
1. Inhalation: Inhalation of high airborne concentration can also irriate mucous membranes, cause headaches, sleepiness, nausea, confusion, loss of consciousness, digestive, and visual disturbances and even death.
2. Skin Contact: methanol is a mild to moderate eye irritant. High vapour concentration or liquid contact with eyes causes irritation, tearing and burning.
3. Ingestion: Swallowing even small amount of methanol could potentially cause blindness or death. Effects of sub lethal doses may be nausea, headache, abdominal pain, vomiting and visual disturbances ranging from blurred vision to light sensitivity.
3.7.2 Effects of long – term Exposure:
Repeated exposure by in halation or absorption may cause systemic poisoning, brain disorder, impaired vision and blindness. In halation may worsen conditions such as emphysema or bronchitis. Repeated skin contact may dermal irritation, dryness and cracking.
First Aid Measures
1.Eye Contact: remove contact lenses if worn. In case of contact, immediate flash eyes with plenty of clean running water for at least 15 minutes, lifting the upper and lower eyelids occasionally.
2.Skin Contact: in case of contact, remove contaminated clothing. In a shower, wash affected areas with soap and water for at least 15 minutes. Seek medical attention if irritation occurs or persists. Wash clothing before reuse.
3.Inhalation: Remove to fresh air, restore or assists breathing if necessary. Obtain medical attention.
4.Ingestion: Swallowing methanol is potentially life threatening. On set of symptoms may be delayed for 18 to 24 hours after digestion. If conscious and medical aid is not immediately available, don’t induce vomiting. In actual or suspected cases of ingestion, transport to medical facility immediately.
5.Handling and Storage
Handling Procedures: No smoking or open flame in storage, use or handling areas. Use explosion proof electrical equipment. Ensure proper electrical grounding procedures are in place.
6.Storage: store in totally enclosed equipment, designed to avoid ignition and human contact. Tanks must be grounded, vented and should have vapour emission controls. Tanks must be diked. Avoid storage with incompatible materials. Anhydrous methanol is non – corrosive to must metals at ambient temperatures except for lead, nickel, monel, cast iron and high silicon iron. Coating of copper, zinc (including galvanized steel), or aluminum are unsuitable for storage.
These materials may be attached slowly by the methanol. Storage tanks of welded construction are normally satisfactory. They should be designed and built in conformance with good engineering practice for the material being stored. While plastics can be used for short term storage, they are generally not recommended for long – term storage due to deterioration effects and the subsequent risk of contamination.
CHAPTER FOUR
4. Results and Discussion
This section describes the results obtained from the simulation of process starting with the synthesis gas part, the methanol synthesis part procedure applied on the process.
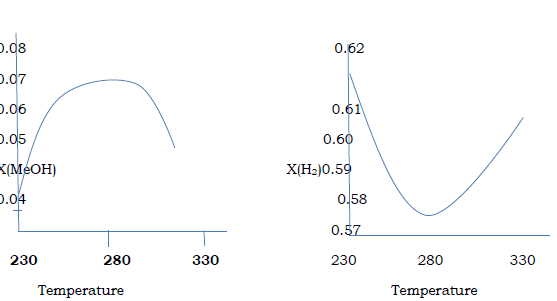 Figure.2 . steady state concentration with Varying temperature (P = 76.98 bar)
Figure.2 . steady state concentration with Varying temperature (P = 76.98 bar)
The show diagram in the above the concentration of methanol and hydrogen changes with the temperature of process. Although the diagram shows the optimum temperature to be around 2700C, this temperature can affect the activity of the catalyst in the also will lead to deactivation of the catalyst within a short period of time. Instead a temperature of 2500C was used in the simulation.
Temperature Variation
The influence of temperature on the mole fraction is given in figure . . for
catalyst C and the standard measurement (P = 20 bar, H2/Co2 ratio = 3,
Q initial= 600 Nml.min-1 and gas hourly space velocity Sv = 4500h-1 ).
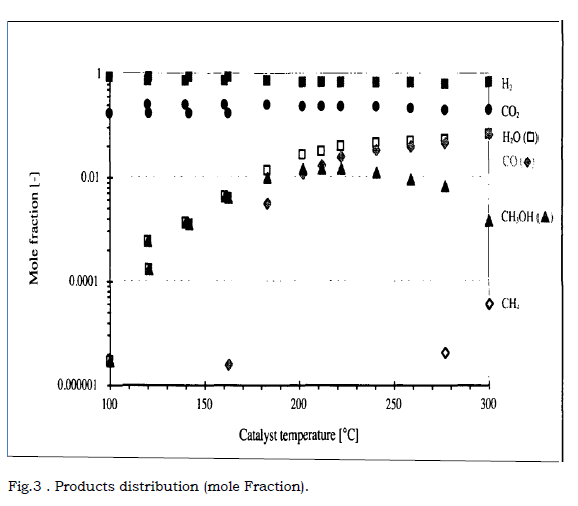 As we see in chemical reactions, temperature is an important factor. Product selectively and activity, as well as catalyst, the three main products resulting from Co2 hydrogenation are methanol, carbon monoxide and water. Methane is also formed at higher temperature is above 2700C produced negligible.
I decided upon this experiment condition although high temperature favour the formation of light alkanes, dimethyl ether and esters. Co, H2O and CH4 concentration increase as the temperature is raised. CH3OH concentration is greatest at about 2200C.
As we see in chemical reactions, temperature is an important factor. Product selectively and activity, as well as catalyst, the three main products resulting from Co2 hydrogenation are methanol, carbon monoxide and water. Methane is also formed at higher temperature is above 2700C produced negligible.
I decided upon this experiment condition although high temperature favour the formation of light alkanes, dimethyl ether and esters. Co, H2O and CH4 concentration increase as the temperature is raised. CH3OH concentration is greatest at about 2200C.
CHAPTER FIVE
5.Conclusion
Methanol is a colour less liquid that may exposed when exposed to an open flame. It occurs naturally in wood and in volcanic gases. Methanol is also a product of decaying organic material. It is produced in large amounts (approximately 1.3 billion gallons 1992) by 13 companies in united states.
Methanol is used in a variety of industrial applications. Its largest use is as a raw material for the production of methyl t-butyl ether (MTBE) a gasoline additive. It is also used in the production of formaldehyde, aceticacid, chloromethanes, methyl methacrylate, methylamines, dimethyl terephthalate, and as solvent or antifreeze in paint strippers, aerosol spray paints, wall paints, carburetor cleaners and car wind shield washer compounds.
Health effects of methanol properties toxicity are well understood. Human exposure to methanol can occur via the inhalation, ingestion or dermal contact path ways. Identified human illnesses associated with optic nerve damage and central nervous system (CNS) effects.
I concluded Co2 mitigation presents both challenges and opportunities to the world for sustainability of energy and environment. Co2 mitigation should focus on the utilization of Co2, for example useful chemicals from Co2 and the Co2 recycling combined with renewable energy to conserve carbon sources.
Therefore, advance of the Co2 including its activation and utilization will be necessary component of the development of sustainable economy in our society.
Reference
1.Akah P.A And Okafor C.l.Blood Sugar Lowering Effect Of Vernonia Amygdalina In An Experimental Rabbit Model, Phytothempy, 1992.
2.Centi G, Perathoner S, Opportunities And Prospects In The Chemical Recycling Of Carbon dioxide To Fuels.
3.D.J. souk up, Methanol In Gasoline, Internal Communication, 11 August 1970.
4.E.S. Starkman, etal, “ Alternative Fuels For Control Of Engine Emission”,Journal Of Air Pollution Control Association, February 1970.
5.Fujitani T, etal ; Methanol Synthesis From Co2 And H2 Over Cu/Zn/Ga2O3, Chemistry Letters, 1993.
6.G.H.Graaf, H.S, EJ. Stamhuis And A.A.C.M. Beenackers, (1989), “Intera – Particle Diffusion Limitation In Low – Pressure Methanol Synthesis”, Chemical Engineering Science.
7.H.Araka, K Sayama, Methanol Synthesis From Co2 and H2 Over Supported Copper Zinc Oxide Catalyst, Significant Influence Of Support On Methanol Formation, G.L.et al(ED), New Frontiers In Catalysis, Budapest, (1992).
8.H.Jaffee, etal, Methanol From Coal For The Automotive Market, Feb 1974.
9.J.H.Edwards, Catal. 23 (1995).
10.J.l.li,T.Inui, Appl. Catal, A: General (1996).
11.J.H. DeBoer, (1958), The Structure And Properties Of Porous Materials, Butterworth, London.
12.Jacobs A.Moulijn M.M, And Annelies Van Diepen,(2001),Chemical Process Technology, John Wiley And Sons. LTD.
13.K.Fujimoto, Y. Yu, Stud. Surf. Sci. Catal.(1993).
14.king H,Aubert R.E and Herman W.H. Global Burden Of Diabetes, Prevalence, Numerical Estimates And Projection.Diabets Care, 1998.
14.M.Sahibzada, D.Chadwick, I.s. Metcalfe, Stud, Surf. Sci. Catal, (1997).
15.M.Sahibzada, D.Chadwick,I.S.Metcalfe, Stud, Surf.Sci.Catal, ( 1997).
16.M.M.Halmann, (1993),Chemical Fixation Of Carbon dioxide: Methods For Recycling Co2 Into Useful Products (CRC PRESS INC, Boca Raton,florida,ed. ).
17.Ortelli E.E, Wambachj, wokaum A, Methanol Synthesis Reactors Over A CuZr Based Catalyst Investigated Using Periodic Variations Of Reactant Concentrations. (2001).
18.Presentation Made At The Methanol As An Alternate Fuel, Engineering Foundation Conference, Henniker, NH July 1974.
19.Pontez F.liebner W. Gronemann V., Rothaemel M, Ahlers Co2 based methanol and DME – Efficient technologies for industrial scale production, 2011.
20.R.E. Train, Statement Before The Subcommittee On Priorities And Economy In Government, Joint Economic Committee, May 1974.
21.R.S. Krik and D.S. Othmer, Encyclopedia Of Chemical Technology, New York Inter Science Encyclopaedia, 1956.
22.S.PS. Andrew, Paper 12, Post Congress Symposium, 7th International Congress On Catalysis, Osaka, (1980).
23.Sigurd Skogestad,(2000), Plant Wide Control: The Search For The Self Optimizing Control Structure. “Journal Of Process Control”.
24.S.Skogested And I. Postlethwaite, (2005), Multivariable Feedback Control, 2nd Edition, John Wiley And Sons Ltd.
25.Stocznski j. Grabowski R. et al, Effect Of Mg And Mn Oxide Addition On Structural And Adsorptive Properties Of Cu/Zno/Zro2 Catalysts For The Methanol Synthesis From Co2. (2003).
26.Skrzypekz, Lachoweska M,serafin D; Methanol Synthesis From Co2 and H2: Dependence Of Equilibrium Conversions And Exit Equilibrium Concentration Of Components On The Main Process Variables. Chemical Engineering Science,1990.
27.Steinberg,M,(1996), The Carnol System For Methanol Production And Co2 Mitigation From Coal – Fired Power Plants And The Transportation Sector, US Department Of Energy.
28.Sherma R.D, Sarkhar D.K and Hazra MB,Toxicological Evolution Of Fenugreek Seeds, Along Term Feeding Experiment In Diabatic Patients, Phytother. 2010.
29.T.Inui, T.Takeguchi, A.Kohama, K.Kitagawara,Stud.Surf.Sci.Catal, (1993).
30.T.Inui,K.Kitagawa, T.Takeguchi, T.Hagiwara, Y.Makino Appl. Catal.A: General, (1997).
31.T.inui, T.Takeguchi, Catal. (1991).
32.U.Akba, Y.Nakamura, K.Suga,M.Fujihira, Thin Solid Films (1992).
33.Undie, As and Akubue P.I. Pharmacological Evaluation Of Dioscon Dumentorium Tuber Used In Traditional Antidiabetic Therspy.j.Ethnopharmacol, 1986.
34.V.M. Cherednichenko, Korpova, Physico – Chemical Institute, Moscow, (1953).
35.V.G. Samoilovich, V.I. Gibalov, K.V.Kozlov,(1989) “Physical Chemistry Of The Barrier Discharge”, Moscow State University.
36.Wu – Hsun Cheng And Harold H.Kung Ed,(1994), Methanol Production And Use Chemical Industries, CRC Press.
37.W.D. Harris and RR.Davison, Methanol from Coal oil and gas journal, 17 Dec 1973.
38.W.R.A.M. Robinson, (1989),“Structure And Activity Of Copper Containing Methanol Synthesis Catalysts”. University Of Amsterdam Thesis.
39.Yoo ch, lee D – w, Kim M-s, Moon Dj, lee, The Synthesis Of Methanol From Co/Co2/H2 Gas Over Cu/Ce, XZrO2 Catalysts. Journal Of Molecular Catalysis. (2013).